Additional information
Yellow Powder
949916-82-9
ACI-020820
>300°C
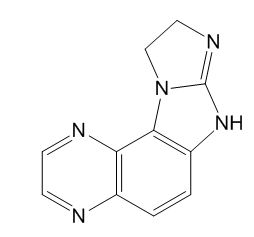
C11H9N5
211.22
Ambient
2-8°C
ocular hypertension
Pharmaceutical Impurity Standards
9,10-Dihydro-7H-imidazo[2',1':2,3]imidazo[4,5-f]quinoxaline
9,10-Dihydro-7H-imidazo[2',1':2,3]imidazo[4,5-f]quinoxaline
7H-Imidazo[1?,2?:1,2]imidazo[4,5-c]-1,5-naphthyridine, 9,10-dihydro;3,8,11,13,16-Pentaazatetracyclo[8.6.0.0[2,7].0[12,16]]hexadeca-1(10),2,4,6,8,11-hexaene
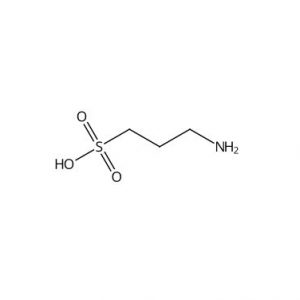
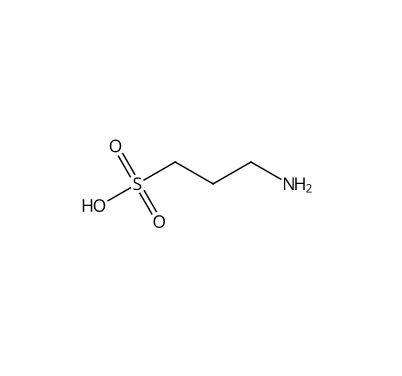
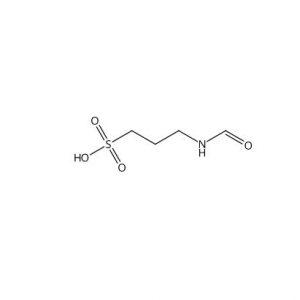
![Ethyl (3R,5S,6E)-7-[4-(4-fluorophenyl)-2-(N-methylmethylsulfonamido)-6-(propan-2-yl)pyrimidin-5-yl]-3,5-dihydroxyhept-6-enoate](https://staging.analyticachemie.in/wp-content/uploads/1A06130-150x150.png)
![2-sec-butyl-4-{4-[4-(4-methoxyphenyl)piperazin-1-yl]phenyl}-2H-1,2,4-triazol-3(4H)-one](https://staging.analyticachemie.in/wp-content/uploads/1A06380-150x110.png)
![Abiraterone Isopropyl Ether (25 mg) (3-((3S,8R,9S,10R,13S,14S)-3-isopropoxy-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15-dodecahydro-1H-cyclopenta[a]phenanthren-17-yl)pyridine; 3beta-Isopropoxy-17-(pyridin-3-yl)androsta-5,16-diene)](https://staging.analyticachemie.in/wp-content/uploads/image-1-150x145.png)

