Additional information
White Powder
1062-09-05
ACI-022611
251.4-253.3°C
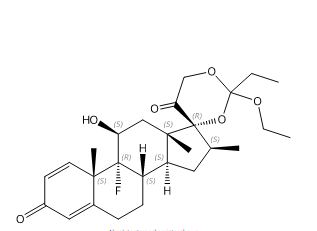
C27H37FO6
476.58
Ambient
2-8°C
Corticosteroids
Pharmaceutical Impurity Standards
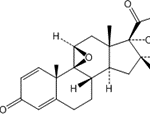
(11ß,16ß)-17,21-[(1-ethoxypropylidene)bis(oxy)]-9-fluoro-11-hydroxy-16-methyl-pregna-1,4-diene-3,20-dione
(11ß,16ß)-17,21-[(1-ethoxypropylidene)bis(oxy)]-9-fluoro-11-hydroxy-16-methyl-pregna-1,4-diene-3,20-dione
Pregna-1,4-diene-3,20-dione, 17,21-[(1-ethoxypropylidene)bis(oxy)]-9-fluoro-11-hydroxy-16-methyl-, (11?,16?)
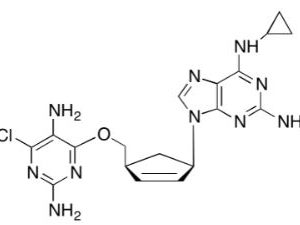
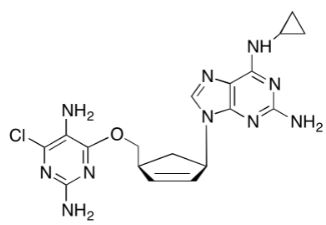
![Ethyl (3R,5S,6E)-7-[4-(4-fluorophenyl)-2-(N-methylmethylsulfonamido)-6-(propan-2-yl)pyrimidin-5-yl]-3,5-dihydroxyhept-6-enoate](https://staging.analyticachemie.in/wp-content/uploads/1A06130-150x150.png)
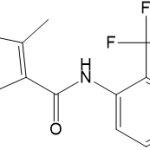
![2-sec-butyl-4-{4-[4-(4-methoxyphenyl)piperazin-1-yl]phenyl}-2H-1,2,4-triazol-3(4H)-one](https://staging.analyticachemie.in/wp-content/uploads/1A06380-150x110.png)
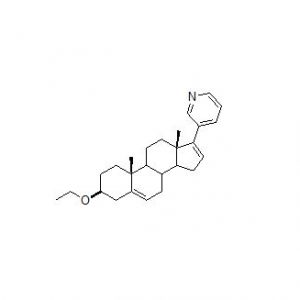
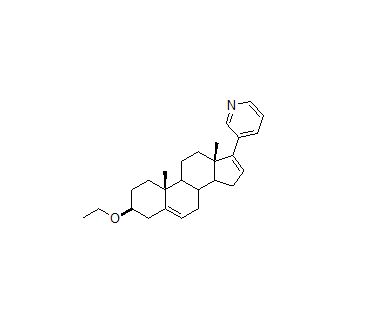
![Abiraterone Isopropyl Ether (25 mg) (3-((3S,8R,9S,10R,13S,14S)-3-isopropoxy-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15-dodecahydro-1H-cyclopenta[a]phenanthren-17-yl)pyridine; 3beta-Isopropoxy-17-(pyridin-3-yl)androsta-5,16-diene)](https://staging.analyticachemie.in/wp-content/uploads/image-1-150x145.png)

