Additional information
Grey colour Powder
191939-34-1
ACI-021717
–
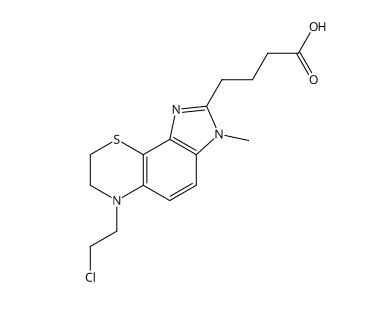
C16H20ClN3O2S • HCl
390.33
Ambient
2-8°C
Chemotherapy
Pharmaceutical Impurity Standards
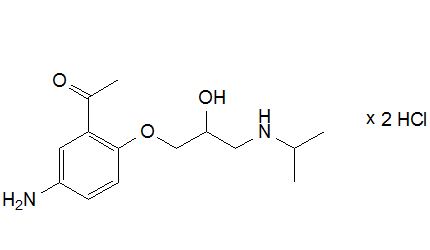
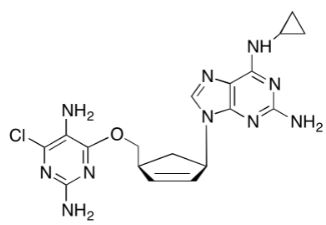
4-(6-(2-Chloroethyl)-3-methyl-3,6,7,8-tetrahydroimidazo[4',5':5,6]benzo[1,2-b][1,4]thiazin-2-yl)butanoic Acid Hydrochloride
4-(6-(2-Chloroethyl)-3-methyl-3,6,7,8-tetrahydroimidazo[4',5':5,6]benzo[1,2-b][1,4]thiazin-2-yl)butanoic Acid Hydrochloride
Imidazo[4,5-h][1,4]benzothiazine-2-butanoic acid, 6-(2-chloroethyl)-3,6,7,8-tetrahydro-3-methyl
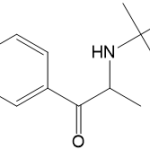
![Ethyl (3R,5S,6E)-7-[4-(4-fluorophenyl)-2-(N-methylmethylsulfonamido)-6-(propan-2-yl)pyrimidin-5-yl]-3,5-dihydroxyhept-6-enoate](https://staging.analyticachemie.in/wp-content/uploads/1A06130-150x150.png)
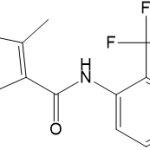
![2-sec-butyl-4-{4-[4-(4-methoxyphenyl)piperazin-1-yl]phenyl}-2H-1,2,4-triazol-3(4H)-one](https://staging.analyticachemie.in/wp-content/uploads/1A06380-150x110.png)
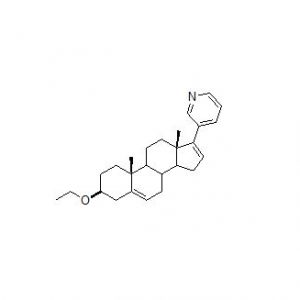
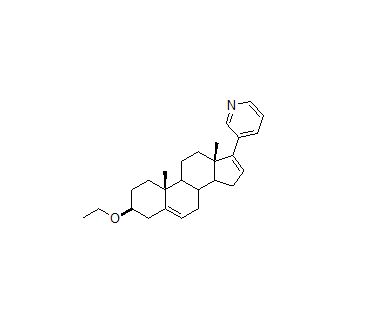
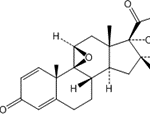
![Abiraterone Isopropyl Ether (25 mg) (3-((3S,8R,9S,10R,13S,14S)-3-isopropoxy-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15-dodecahydro-1H-cyclopenta[a]phenanthren-17-yl)pyridine; 3beta-Isopropoxy-17-(pyridin-3-yl)androsta-5,16-diene)](https://staging.analyticachemie.in/wp-content/uploads/image-1-150x145.png)

