Additional information
Free Base-112110-48-2
White Powder
NA
ACI-023404
66.1-67.0°C
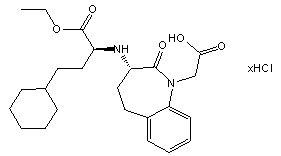
C24H35ClN2O5
467
2-8°C
Antidiabetic
Pharmaceutical Impurity Standards
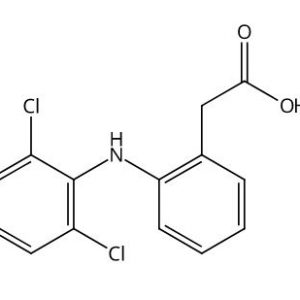
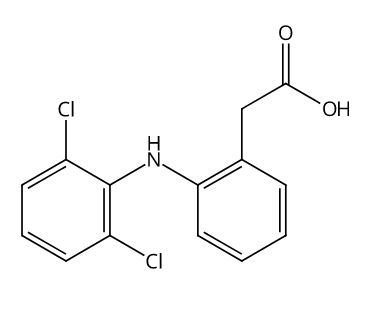
(3-(1-Ethoxycarbonyl-3-cyclohexyl-(1S)-propyl)amino-2,3,4,5-tetrahydro-2-oxo-1H-1-(3S)-benzazepine-1-acetic acid monohydrochloride)
(3-(1-Ethoxycarbonyl-3-cyclohexyl-(1S)-propyl)amino-2,3,4,5-tetrahydro-2-oxo-1H-1-(3S)-benzazepine-1-acetic acid monohydrochloride)
1H-1-Benzazepine-1-acetic acid, 3-[[3-cyclohexyl-1-(ethoxycarbonyl)propyl]amino]-2,3,4,5-tetrahydro-2-oxo-, [S-(R*,R*)]
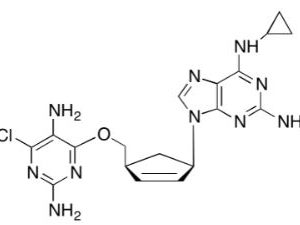
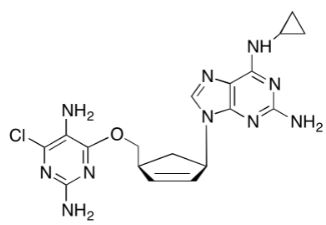
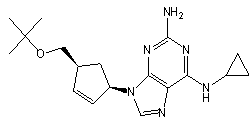
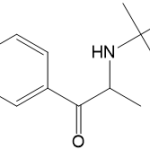
![Ethyl (3R,5S,6E)-7-[4-(4-fluorophenyl)-2-(N-methylmethylsulfonamido)-6-(propan-2-yl)pyrimidin-5-yl]-3,5-dihydroxyhept-6-enoate](https://staging.analyticachemie.in/wp-content/uploads/1A06130-150x150.png)
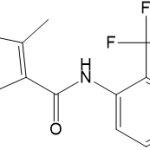
![2-sec-butyl-4-{4-[4-(4-methoxyphenyl)piperazin-1-yl]phenyl}-2H-1,2,4-triazol-3(4H)-one](https://staging.analyticachemie.in/wp-content/uploads/1A06380-150x110.png)
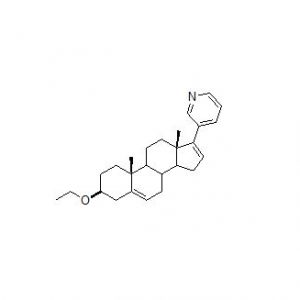
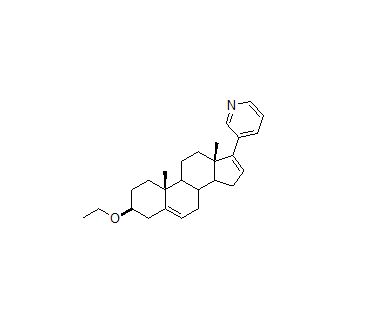
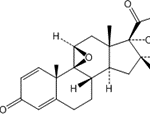
![Abiraterone Isopropyl Ether (25 mg) (3-((3S,8R,9S,10R,13S,14S)-3-isopropoxy-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15-dodecahydro-1H-cyclopenta[a]phenanthren-17-yl)pyridine; 3beta-Isopropoxy-17-(pyridin-3-yl)androsta-5,16-diene)](https://staging.analyticachemie.in/wp-content/uploads/image-1-150x145.png)

