Additional information
Current Lot Information
Current Lot:
F231W0
CAS RN®
252964-68-4
Harmonized System (HS) Code *:
98020000
UN No:
N/A
NDC No:
N/A
Molecular Formula
C23H29N5O2
Container Type:
VIAL
Base Control Substance (substance %):
N/A
Product Information
SDS:
USP Certificates/Product Information Sheets and Valid Use Dates
|
Lot No |
USP Certificates/Product Information Sheets and Valid Use Dates |
Valid Use Date |
Country of Origin* |
Material Origin* |
|---|---|---|---|---|
|
F231W0 |
No data available |
See Product Information Sheet |
India |
No data available |
|
Lot No F231W0 |
USP Certificates/Product Information Sheets and Valid Use Dates No data available |
|
Valid Use Date See Product Information Sheet |
|
|
Country of Origin* India |
|
|
Material Origin* No data available |
* Certain Material Origins (i.e. Animal, Plant, Fish) may require special country importation requirements. USP recommends you contact your country competent authorities to determine if any certifications, permits or licenses may be required prior to ordering. Material Origins are found within the Product under Origin Information.
*Disclaimer:
* The Harmonized System (HS) code provided on this webpage is for informational purposes only and is subject to change without notice. The exporter and/or importer of record is responsible for determining the accuracy of items at the time of export/import per U.S. regulations. USP is not responsible for the accuracy or completeness of the information furnished.
* USP procures materials worldwide and most foreign materials do not undergo a fundamental change during the packaging process at USP that would substantially transform the item resulting in a country of origin change from the foreign origin to the United States. Under the Rules of Origin the original foreign country of origin would remain.

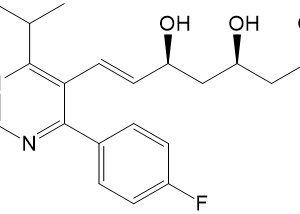
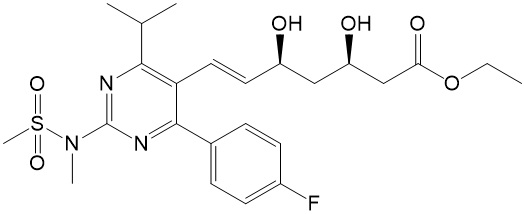
![Ethyl (3R,5S,6E)-7-[4-(4-fluorophenyl)-2-(N-methylmethylsulfonamido)-6-(propan-2-yl)pyrimidin-5-yl]-3,5-dihydroxyhept-6-enoate](https://staging.analyticachemie.in/wp-content/uploads/1A06130-150x150.png)
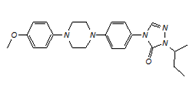
![2-sec-butyl-4-{4-[4-(4-methoxyphenyl)piperazin-1-yl]phenyl}-2H-1,2,4-triazol-3(4H)-one](https://staging.analyticachemie.in/wp-content/uploads/1A06380-150x110.png)
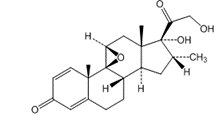
![Abiraterone Isopropyl Ether (25 mg) (3-((3S,8R,9S,10R,13S,14S)-3-isopropoxy-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15-dodecahydro-1H-cyclopenta[a]phenanthren-17-yl)pyridine; 3beta-Isopropoxy-17-(pyridin-3-yl)androsta-5,16-diene)](https://staging.analyticachemie.in/wp-content/uploads/image-1-150x145.png)

