- Labetalol(1)
- Lacosamide(2)
- Lamivudine(1)
- Lamotrigine(1)
- Lansoprazole(3)
- Leflunomide(1)
- Lenalidomide(3)
- Levetiracetam(1)
- Lidocaine(1)
- Linagliptin(1)
- Linezolid(2)
- Lisinopril(2)
- Loperamide(1)
-
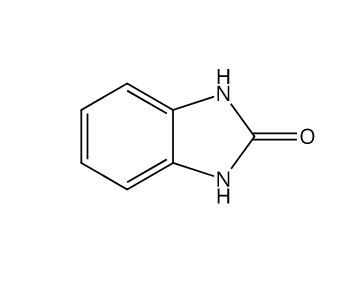
Lansoprazole Imp. D (EP)
Catalogue No.
ACI-120204
CAS No.
615-16-7
Molecular Formula
C7H6N2O
-
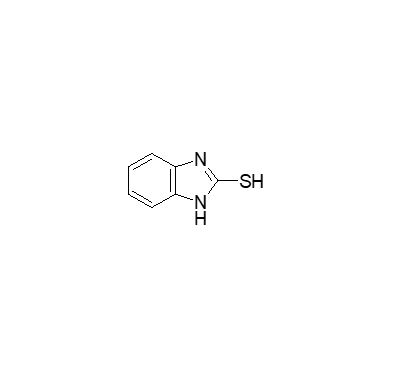
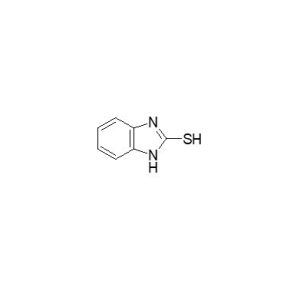
Lansoprazole Imp. E (EP)
Catalogue No.
ACI-120205
CAS No.
583-39-1
Molecular Formula
C7H6N2S
-
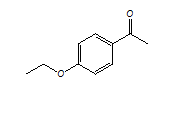
Lansoprazole Impurity
Catalogue No.
ACI-120214
CAS No.
1676-63-7
Molecular Formula
C10H12O2
Got any Queries?
We value your curiosity and strive to provide you with all the information you need. If you have any questions or need further details about our products/services, company, or any other topic related to our website, feel free to reach out to us. Our team is here to assist you promptly.
Contact Us